How does xcube.bio work?
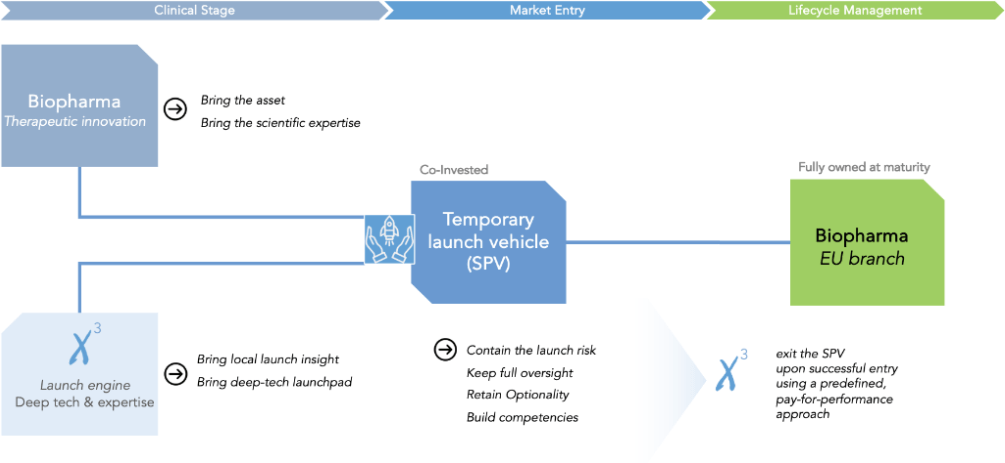
Combining strengths during the risky Market-entry period using a temporary launch vehicle, in order to build a fully functioning European organization
An optimal sharing of value with similar risk profile as a Licensing agreement
An entity with full oversight and mid-term control
Optionality protected for innovator: entity buy back, carry-on, switch to licensing, exit.
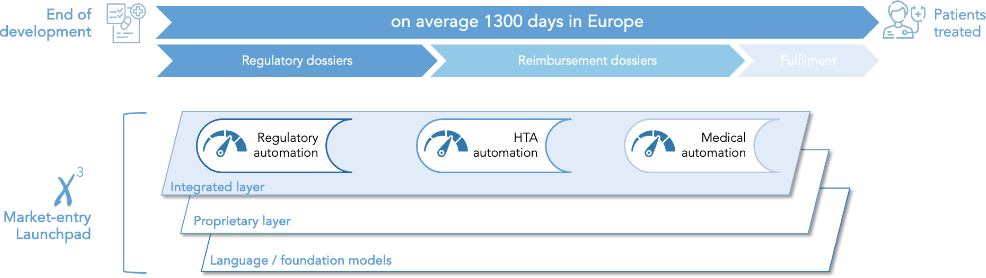
Rethinking market-entry processes with deep-tech lenses
Automation drives triple impact: shorter time to patient, lower upfront cost and faster revenue generation
From the decision to file in Europe to the commercial patient, European regulator, national payers and hospitals set up processes and require to build up precise dossiers for each indications. Put one after the other, completion of those takes take on average 1300 days.
Xcube.bio leverages the power of deep-tech to rewire market entry processes.

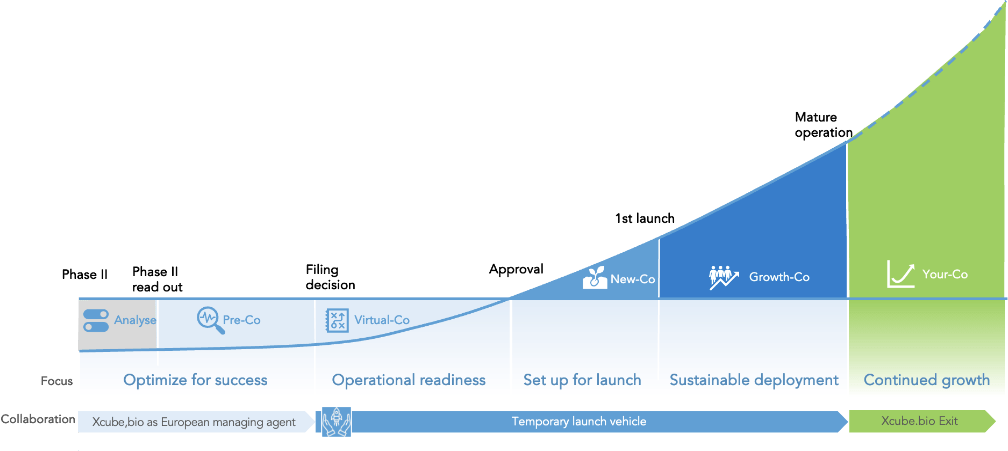
Clarify the commercial ambition. Understand the patient journey, formalize the anticipated commercial features of the asset and design the go-to-market model to succeed in markets.
Xcube.bio brings hands-on expertise to design the commercial success

Prepare for the future. Make the right early decision to upgrade the asset from “approvable” to “marketable”. Execute the initial engagement with local stakeholders, potential partners and suppliers. Optimize the commercial potential and validate the investment needs with field input.
Xcube.bio delivers day-to-day management, decision support and targeted expertise as a virtual liaison office

Time to initiate action. Upgrade engagement with market stakeholders, solidify partner and supplier relationships, finalize plans for commercial processes and systems, and scout for talents.
Xcube.bio undertakes the initial tasks until Marketing Authorization (MA) as a Virtual prelaunch squad fully syncs with innovator

The rubber hits the road. Commercial operational entities are created, systems and distribution are deployed, and key recruits are onboarded.
Xcube.bio ensures legal, organizational, and personnel readiness as a co-invested partner

Deliver the launch. Progressively deploy across the target territories, ramp up customer-facing teams, and deliver outcomes for patients.
The commercial entity executes the launch plan under the scientific supervision of the Innovator and operational advisory of Xcube.bio.

Time for xcube.bio to exit. The mature commercial entity is fully owned by the innovator. Systems and processes are integrated with the innovators’. The commercial entity continues its growth journey.
Xcube.bio is hands-off. It’s all up to the innovator now, standing as a full-scale commercial-stage biotech.